Cornyn, Sinema, Hyde-Smith, Jones Introduce Kidney Dialysis Innovation Bill
 U.S. Senators Kyrsten Sinema (D-AZ), Cindy Hyde-Smith (R-MS), Doug Jones (D-AL) and I yesterday introduced the Patient Access to ESRD New Innovative Devices Act, legislation that calls for the Secretary of the U.S. Department of Health and Human Services to make changes to the Medicare payment program for dialysis to allow for new medical devices that can improve dialysis treatment and patient outcomes.
U.S. Senators Kyrsten Sinema (D-AZ), Cindy Hyde-Smith (R-MS), Doug Jones (D-AL) and I yesterday introduced the Patient Access to ESRD New Innovative Devices Act, legislation that calls for the Secretary of the U.S. Department of Health and Human Services to make changes to the Medicare payment program for dialysis to allow for new medical devices that can improve dialysis treatment and patient outcomes.
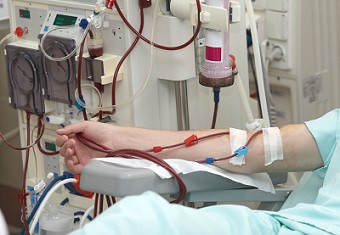
Dialysis is a life-saving technology used by millions of Americans, but there are more advancements that can be made to increase its effectiveness and patient quality of life. This legislation would promote innovation and make it easier for those with end-stage renal disease to be treated with updated medical devices.
“We’re lowering the cost of health care for Arizonans and giving Arizona manufacturers the ability to grow, innovate, and create life-saving treatments for patients and families who have few options,” said Sen. Sinema.
“For too long, Medicare rules have effectively held up research and innovation on new dialysis technologies, which has real consequences for many people in Mississippi and elsewhere who suffer from kidney disease,” Sen. Hyde-Smith said. “We want to update Medicare payment options to instead incentivize the development and use of improved dialysis options that can improve the quality of life for patients with renal failure issues.”
“This legislation will spur innovation in dialysis, which will help improve outcomes and quality of life for the nearly 12,000 Alabamians who are currently on dialysis,” said Sen. Jones.
Background:
The Patient Access to ESRD New Innovative Devices Act would create a three-year temporary add-on payment adjustment for new medical devices that provide meaningful clinical improvement for the diagnosis, treatment, or management of end-stage renal disease.
There are approximately 400,000 Medicare beneficiaries with end-stage renal disease, making up one percent of the Medicare population but accounting for approximately seven percent of all Medicare spending.
Unlike other disease states, there has been a significant lack of innovation in the devices used to treat kidney disease. Most people with end-stage renal disease (ESRD) receive in-center hemodialysis, a process that has changed little in the decades since it was invented. One data point that bears out the lack of innovation is a comparison of clinical trials in the field of nephrology versus other disease states. According to the Journal of the American Society of Nephrology, from 1966 to 2002, the number of randomized controlled trials in the area of nephrology was lower than all other specialties of internal medicine. (Compare 5335 RCTs in hematology to 27109 in cardiology.)
The only real innovation in recent decades in the ESRD space was drug-related, not device related. Specifically, the development of the anemia drug erythropoietin represented a big step forward in kidney care. But as a 2017 Milken Institute study underscored, further discovery of drugs to advance the treatment of kidney disease is unlikely. Specifically, the report concluded that “[t]he clinical development landscape [for kidney disease] is incredibly barren because of the paucity of clinically relevant molecules to target therapeutically.”
The federal government has recognized the need to spur device innovation in treating kidney disease. The FDA awarded the FDA’s Expedited Access Pathway to the wearable artificial kidney in 2015, and in April of 2018, HHS announced the creation of Kidney X, a public-private partnership to promote innovation in treating kidney disease. In spite of this focus, there has only been one de novo application related to dialysis submitted to the FDA.